Injection Port – Self-Sealing IV Port
Self-sealing injection port for IV bags. Sterile, leak-proof, and autoclavable. Ideal for safe drug admixture in parenteral packaging.
Injection Port
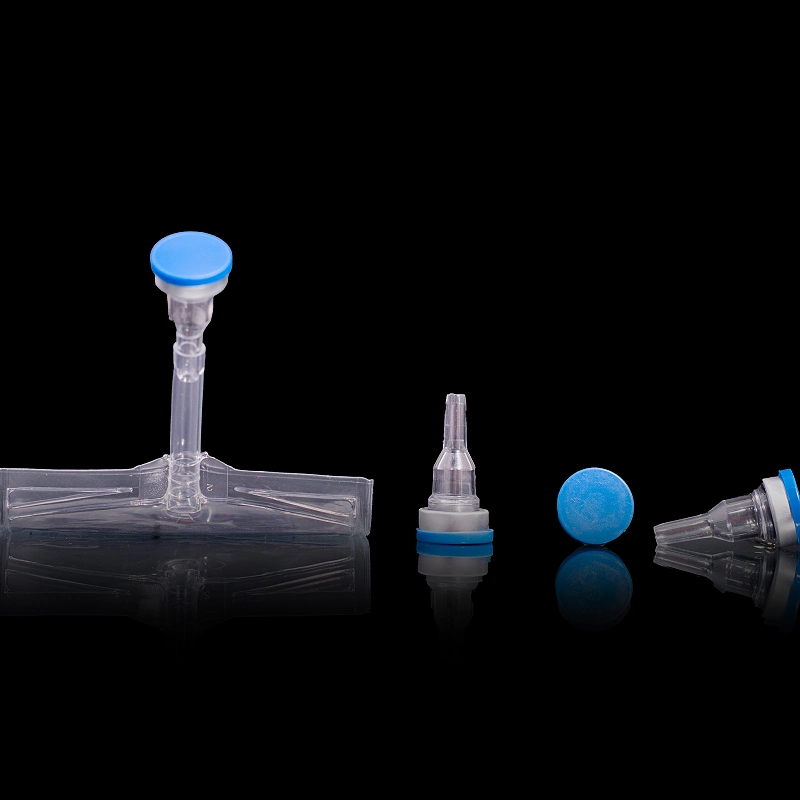
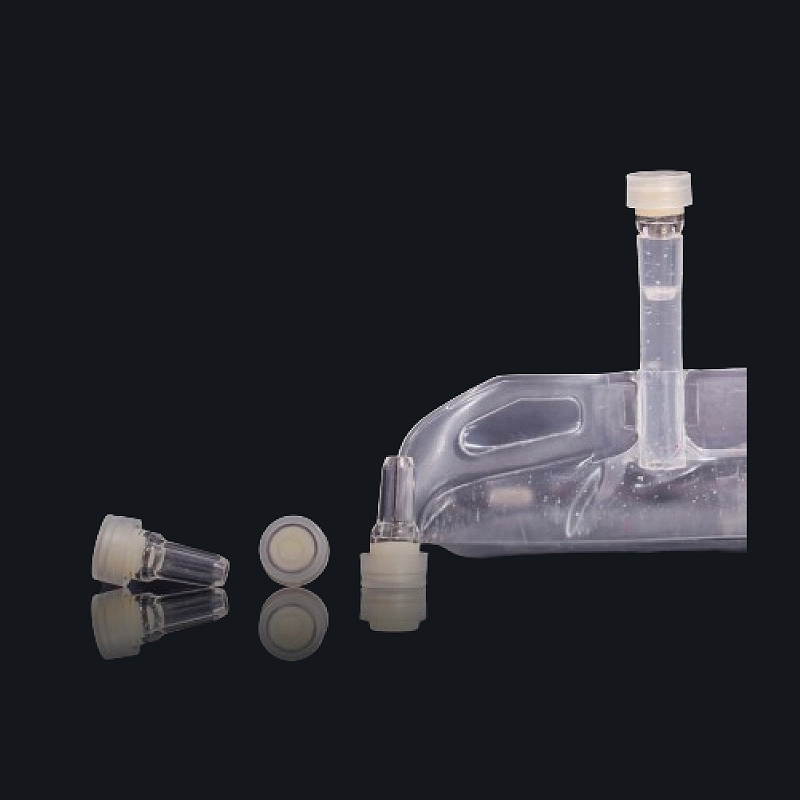
The Injection Port for IV Bags is a self-sealing, sterile access port designed for safe admixture of parenteral drugs into IV fluids using standard syringes. It enables direct, contamination-free drug injection while preserving the integrity of the IV solution before and after administration.
Made with medical-grade polypropylene (PP) and a rubber elastomer sealing ring, the port supports needle retraction sealing, leak-proof performance, and long-term sterility. Manufactured under ISO Class 7 cleanroom standards, this injection port is ideal for hospitals, OEM pharma lines, and emergency infusion setups.
Injection Port for IV Bags – Specifications
Product Type: Self-Sealing Injection Port
Application: Parenteral drug admixture into IV bags
Material: Medical-grade Polypropylene (PP) + Rubber Elastomer
Sterilization: Compatible with Gamma and ETO sterilization
Design: Self-sealing, needle-pierceable, resealable
Sealing Function: Leak-proof, needle retraction closure
Facility Compliance: ISO Class 7 cleanroom, ISO 9001:2015, WHO-GMP certified
Compatibility: IV bags, flexible parenteral containers, and LVP systems
Submit Your Inquiry
It allows medications to be injected safely into IV solutions without breaching container sterility.
Yes, the rubber stopper reseals after syringe withdrawal.
Yes, it is both autoclavable and Gamma sterilization compatible.
It’s used in hospitals, infusion therapy systems, and in OEM IV bag production lines.
More Products