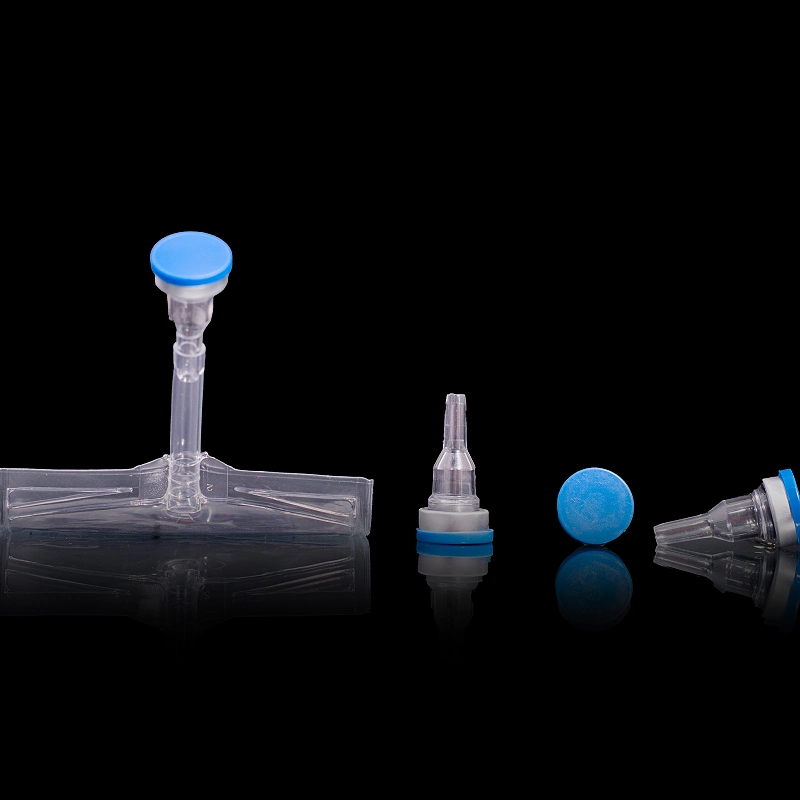
PP Port – Autoclavable Polypropylene Cap
Polypropylene PP Ports for IV bags. Weldable, sterile, and autoclavable. Compatible with BFS/FFS machinery for infusion drug packaging.
PP Ports for IV Bags
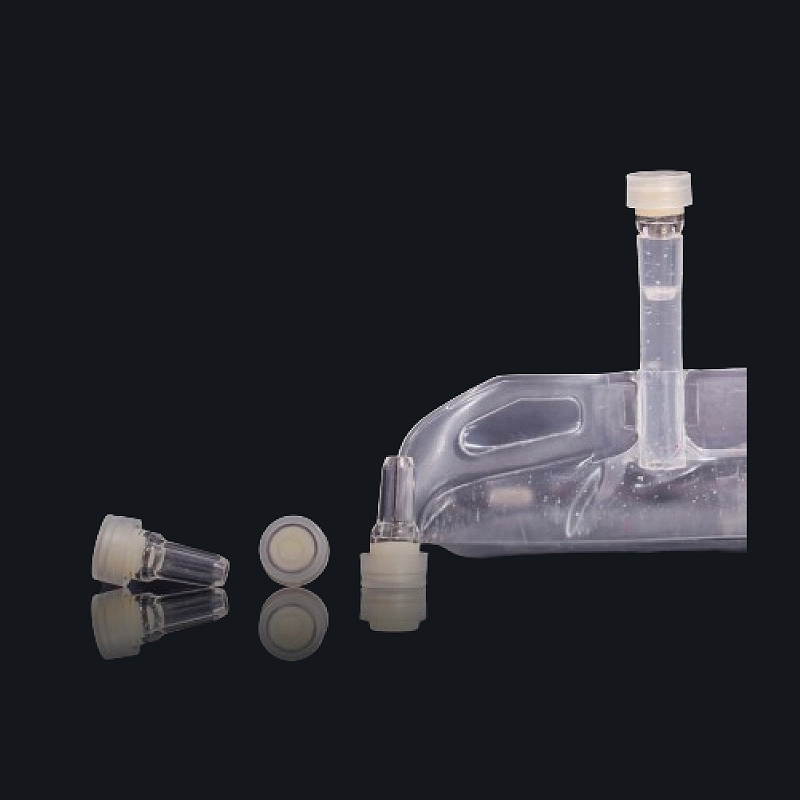
The PP Port for IV Bags is a weldable polypropylene infusion port designed for use with flexible IV containers in parenteral drug delivery. Its compact structure offers chemical resistance, high weld strength, and ensures long-term sterility for safe drug storage and administration in hospital and OEM packaging environments.
Manufactured with medical-grade polypropylene (PP) in ISO Class 7 cleanrooms, this port is built for seamless compatibility with BFS (Blow-Fill-Seal) and FFS (Form-Fill-Seal) production lines. It integrates efficiently with multilayer IV bag films, supporting both automation and sterile packaging standards.

PP Port for IV Bags – Specifications
Product Type: Weldable Polypropylene Infusion Port
Application: IV bag infusion and parenteral drug delivery
Material: Medical-grade Polypropylene (PP)
Design: Weldable base with self-sealing injection channel
Sterilization: Compatible with ETO and Gamma sterilization
Compatibility: BFS/FFS production lines, multilayer IV bag films
Facility Compliance: ISO 9001:2015, WHO-GMP, Class 7 cleanroom
Features: High weld strength, leak-proof, chemically resistant
Submit Your Inquiry
It provides secure, sterile access to IV bags for infusion or injection delivery.
Yes, it’s fully compatible with autoclave, gamma, and ETO sterilization methods.
Absolutely. It’s designed to be weldable using high-speed IV bag manufacturing equipment.
Typically used with 100ml to 1000ml IV bags depending on application and client specification.
More Products